Leadership in enabling and industrial technologies - ICT - InSiDe
Introduction
Integrated Silicon photonics for Cardiovascular Disease monitoring
nr of partners: 10
Coordination: imec - Medtronic
duration: 4 years
Project description
The rationale for Medtronic and partners to propose the InSiDe project is the unmet need of the medical community for reliable, non-invasive, cheap and easy-to-use tools able to identify and characterize different stages of cardiovascular diseases. Solving this need ensures the early adoption of the appropriate therapies, dramatically reduces healthcare costs and importantly, improves patient outcome.
In fact, monitoring arterial stiffness by measurement of the aortic pulse wave velocity has been demonstrated to be a crucial need for the management of hypertensive patients and it is recommended in the European Society of Cardiology Guidelines. In addition, the early identification of arterial stenosis and cardiac contraction abnormalities can be used to drive earlier therapy adoption and to improve patient’s response in cardiac (valvular) disease.
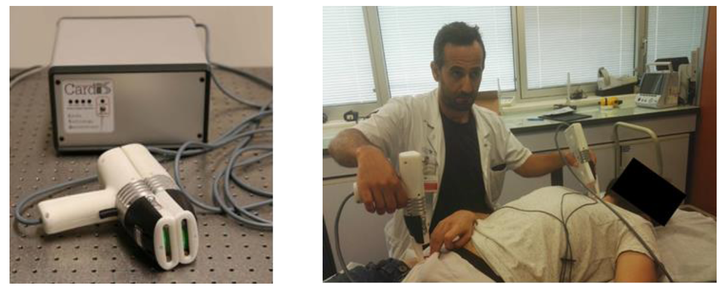
Building on the realizations of the successful CARDIS (H2020-EU.2.1.1.6-644798) project, the objective of InSiDe is to accelerate access to a new diagnostic device, based on silicon photonics technology, able to monitor cardiovascular diseases and to prove its efficacy in driving a timely therapy institution and its related follow-up.
We will:
- Develop an efficient miniaturized laser Doppler interferometer supported by a manufacturable package with integrated imaging optics and by electronics for control of the laser interferometer with onboard near-real time signal processing capability.
- Develop algorithms for translation of the interferometer signals to beat-to-beat measurement results relevant for monitoring and diagnosis of selected cardiovascular parameters
- Prove the device efficacy in multiple clinical feasibility studies inside and outside the consortium
- Outline a path to industrialization and manufacturability
In this way InSiDe will realize a low-cost handheld, robust diagnostic tool, manufacturable in high-volumes. The diagnostic tool gives immediate results for physician’s interpretation.
Objectives
The objective of InSiDe is to provide access for the medical community to a new diagnostic device, based on a silicon photonics integrated homodyne laser interferometer, able to identify and characterize different stages of cardiovascular diseases proving its efficacy to drive an indicated therapy institution and to monitor its follow-up, in order to reduce the healthcare costs and improve patients outcome.
- Objective 1: Development and release of a true handheld wireless clinical investigational device with capability to
o Measure heart-carotid and carotid-femoral PWV as a measure for arterial stiffness and present result on the display of the device.
o Quantify arterial stenosis and present results on the display of the device.
o Record cardiac contraction patterns and present trace with marked fiducial points and timing between those.
o Transfer recorded data to an external computer for scientific purposes and algorithm verification.
- Objective 2: To demonstrate in clinical feasibility studies with the developed clinical investigational device that it is useful for GPs and cardiologists.
Role of Ghent University
• Core-lab activities
• Development, implementation and validation of algorithms for assessment of local PWV/optical auscultation/stenosis detection
Website
to be announced
Contact
Prof. Patrick Segers
Department ELIS
Phone number: +32/9/3323466
E-mail
Prof. Roel Baets
Department INTEC
Phone number: +32/9/3323466
E-mail