Dr. ir. Ashish Kumar
BIOMATH, Model-based analysis and optimisation of (bio)processes
Department of Mathematical Modelling, Statistics and Bioinformatics
Coupure Links 653
B-9000 Gent (Belgium)
Tel.: +32-9-264.59.37
Fax: +32-9-264.62.20
Education: Process engineer
Obtained degree of Doctor in Applied Biological Sciences in 2015
Development of predictive knowledge platform on tabletability for solid dosage manufacturing (PreTAP)
Objectives
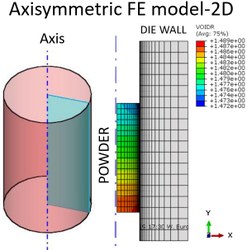
- Understanding the impact of changes in the formulation design space/powder characteristics/process conditions on the Finite element analysis (FEA) prediction of compaction.
- Primary feasibility study to evaluate the application of discrete element method (DEM) approach for powder compaction simulation.
Background
- The most common drug product dosage form is tablet (over 90% of the drug product).
- Development of robust tableting process in a timely manner is still challenging due to lacking fundamental understanding of the process.
- Application of sophisticated process simulation tools can improve mechanistic understanding of the tableting process.
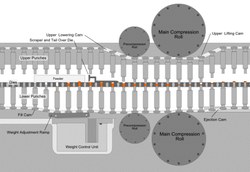

Continuous tableting Compaction Simulator
Methodology
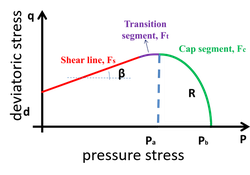
- Obtain the necessary inputs for FEA based on DPC model (Young Modulus, Poisson ratio, cohesion (d), friction angle (β), cap eccentricity (R)) using compaction simulator.
- Carry out FEA simulation for different formulations and punch shape.
- Develop a statistical/mathematical method to quantify goodness of FEA prediction (based on comparing the FEA result and X-Ray CT scan in terms of relative density).