Fluid-solid growth and remodeling of aortic ageing in humans
With increase in computational power, computer models of the cardiovascular system can be considered as virtual patients. These computer models however cannot still accurately explain how tissues behave in response to altered biomechanical load and we hope to explain this using Fluid-Solid growth models. One potential application of these fluid-solid growth models is to study the effect of ageing. Tissues are composite materials that consist of elastin, the protein providing elasticity to soft tissues, which has a half life of 40 years and is considered to play a pivotal role in age-related growth and remodeling of the elastic central vessels as it continually degrades as we grow older. The overall objective is to implement a computational biomechanics fluid-solid growth framework of the Aorta from the ages of 30 to 80 that describes hemodynamic impact on elastin degradation in the artery, geometrical effects like tortuosity and hemodynamic effects like wall shear stresses.
Biomechanical modeling
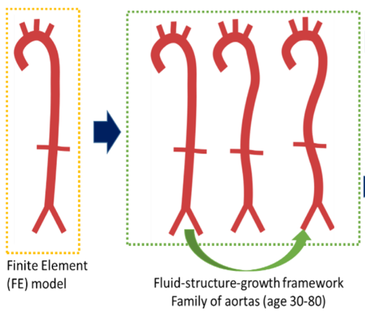
We aim to implement the growth and remodeling algorithm developed in-house at KU Leuven to the baseline geometry of a 30 year old healthy patient obtained from literature. A constrained mixture theory is used to computationally model growth and remodeling of ageing and the mentioned algorithm is implemented in the Finite Element solver ABAQUS. The aim would be to numerically grow the heart from the age of 30 to 80 using the aforementioned theory as illustrated in Figure 1 and to validate results using the Asklepios in-vivo database.
Generating a mesh
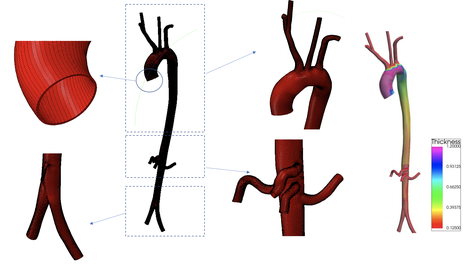
In order to implement the algorithm developed in-house, a structured, hexahedral mesh is necessary to solve the structural equations. This is obtained by generating an initial blocking structure of the aortic surface. Once a good quality surface mesh with quadrilateral elements is obtained, an in-house developed code is used to offset the surface and generate a structured hexahedral mesh ( as shown in Figure 2). A function is used to ensure the thickness of the artery is correspondent to the local radii in the artery as shown in Figure 2.
Hemodynamic modeling
The impact of blood flow and subsequently wall shear-stress are not taken into account in the developed growth and remodeling algorithm. We incorporate this by modelling blood flow inside the artery using the Finite Volume solver FLUENT and implicitly coupling with obtained stresses with the finite element solver Abaqus using the in-house coupling tool CoCoNuT.
Studies on mice model revealed that the mechanical impact of elastin degradation is quantified by reduction of elastic energy compared to stiffness parameters [ref]. So energy metrics can provide useful insight on loss of arterial integrity. It is our aim to relate this biomechanics based elastic energy with hemodynamic based hydraulic energy. Though this novel idea has been theorized, it has never been tested computationally. We also aim to study variation of hemodynamic parameters like time-averaged wall-shear stresses, blood particle residence time, vorticity and their evolution with age.
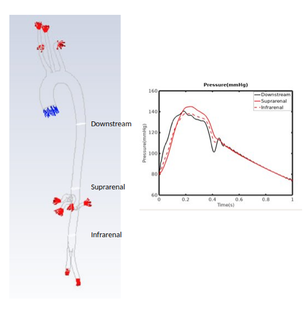
Figure 3 shows pressures obtained using a fluid only simulation of the 30 year old Aorta in the Thoracic, Suprarenal and Infrarenal Aorta.
IBiTech researchers currently active on the project
- Amith Balasubraman (contact)
- Guillermo Fernandez (contact)
- Patrick Segers (contact)
- Joris Degroote (contact)
- Nele Famaey (contact)
Funding sources
FWO project G029819N