Computational modelling of the cardiovascular circulation in zebrafish
Aim and introduction: same goals, new animal model
Zebrafish, probably the eye catcher in the title, but let’s introduce them by starting with something more general. The reason we use computational modelling in this project is twofold, and actually this holds for computational modelling in biomedical engineering in general. Ultimately it allows to (i) reduce the number of (early-stage) experiments and (ii) predict results that are difficult to measure experimentally. Validation of these computational models is of course necessary and because of, a.o., ethical concerns, safety concerns and high throughput problems, humans but also animals closely related to humans are often unfit for early-stage research. That’s why other animal models come into play of which the mouse model is a well-known example. The bioMMeda research group has already established a strong track record where mouse models are used to study cardiovascular diseases.
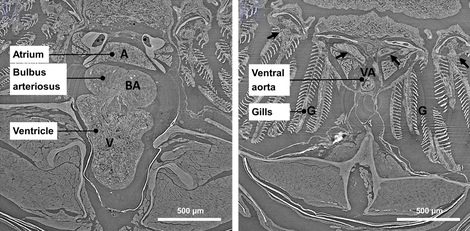
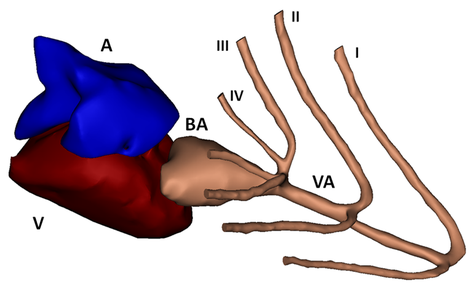
Compared to mice, using zebrafish as an animal model has several advantages: for example easier maintenance, lower cost, easier genetic manipulations and a high throughput potential. Looking at the cardiovascular structures (limited overview in Figures 1, 2 and 3), there are some important anatomic differences. Only two heart chambers are present (1 atrium, 1 ventricle) compared to the 4-chambered human (and mouse) heart. Also, not the lungs but gills and gill vasculature ensure oxygen supply to the fish. In between the ventricle and the (branched) ventral aorta, the bulbus arteriosus is located. The bulbus arteriosus is a peculiar component and serves as an elastic reservoir. Through its buffer working or ‘Windkessel’ effect, pressure peaks are damped and flow towards the delicate gill vasculature is extended. All these differences could seem alarming at first, but zebrafish are 70% genetically similar to humans and they even suffer from the same heart problems. Zebrafish genetic models have already recapitulated numerous human cardio¬vascular disorders.
Another advantage: embryonic and larval zebrafish are transparent which makes them extremely suited to study the development of cardiovascular structures. Next to that, there is already proof that (adult) zebrafish, unlike humans, can regenerate cardiac tissue. This increased the interest to explore the use of zebrafish in cardiovascular research but from a biomedical engineering point of view, modelling of the cardiovascular structures of zebrafish, as has been and is being done for mouse modes, is still lacking.
The general aim of this project is to come up with computational models that capture the cardiovascular circulation of the zebrafish adequately and can be used to phenotype different zebrafish models. Given the increased awareness of the role of mechano-biology in cardiovascular (patho)physiology, a better understanding of the biomechanical factors of the zebrafish circulation may be equally important as our understanding of its biological (genetic) functions. Computational fluid dynamics (CFD), computational solid mechanics (CSM) and fluid-structure interaction (FSI) models will be used for this purpose.
Workflow: a highly interdisciplinary project
Currently, phenotyping of different zebrafish mutants is already being performed at the Center for Medical Genetics of Ghent University (group of Prof. Julie De Backer and dr. Patrick Sips) using, e.g., Pulsed Wave Doppler and B-mode ultrasound measurements. By providing engineering tools and computational models, we aim to describe and compare also other advanced and important measures such as wall shear stress. One of the main challenges in this project is to overcome difficulties due to the very small size of the cardiovascular structures (diameter ventral aorta ̴ 0.1 mm, long axis ventricle ̴ 1 mm). Other partners include Prof. Joris Degroote (computational fluid dynamics, fluid-structure interaction), Prof. Ward De Spiegelaere (corrosion casting), Prof. Matthieu Boone (micro-CT imaging), Prof. Yap Choon-Hwai (4D ultrasound, computational fluid dynamics) and the Paul Scherrer Institute in Switzerland (synchrotron imaging).
IBiTech researchers currently active on the project
Funding sources
As an interdisciplinary research project (IOP), this project is funded by the special research fund (BOF) of Ghent University (BOF.24Y.2018.0011).