Characterization of biophysical properties of solid tumors: towards personalized computational oncology
Biophysical properties of solid tumors
Throughout the years, the poor prognosis of cancer related therapies has been related to the hostile biophysical characteristics of tumor stroma (Figure 1) [1]. On the other hand, chemotherapy has been the most often used therapeutic method, in which the common clinical drug delivery technique is intravenous (IV) or systemic administration in order to reach all cancer cells through the blood circulation. Alternatively, chemotherapy may be applied locally in an organ or tissue, e.g. using the so-called Intraperitoneal drug delivery (IPDD) technique used for peritoneal carcinomatosis, during which the drug directly targets the tumor nodules in the peritoneal cavity. Currently, one of the main limitations of both systemic chemotherapy and IPDD is the fact that drug penetration depth within the tumor is very limited [2]. This issue has been correlated with the elevated interstitial fluid pressure, high tumoral solid stress and stiffness of tumors. However, nowadays, the knowledge on the biophysical characteristics of the tumor microenvironment is still limited due to the fact that there is no standard measurement technique for the above parameters in the majority of solid tumors. Therefore, it is crucial to develop standard non-invasive methods to characterize solid tumors.
Non-invasive quantification of interstitial pressure and drug delivery in solid tumors
The overarching goal of this clinical-translational project is to develop and validate a non-invasive clinical imaging tool that allows to personalize cancer treatment according to the biophysical properties of the tumor stroma. Accordingly, here at BioMMedA and the Experimental surgery lab of UGent, we aim to develop non-invasive methods in order to quantify the IFP, solid stress and stiffness in solid tumors.
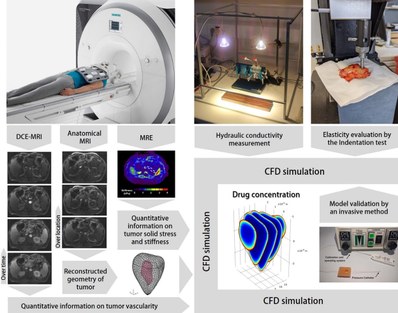
In order to do this, we are implementing magnetic resonance (MR) based techniques such as Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and MR elastography (MRE).
Since very limited literature data are available on the hydraulic conductivity of tumors of the pancreas or ovary in humans, we are also developing other methods for measuring tumor permeability and elasticity using invasive methods to study patient-specific data. These parameters will be measured in vitro on resection specimens of patients who did not undergo neoadjuvant therapy.
Finally, based on 3D tumor geometry reconstructions and experimental datasets, we are developing 3D computational fluid dynamics (CFD) models to realistically simulate the tumor’s interstitial flow in order to generate a personalized-map of drug distribution in the tumor during therapy. Figure 2 illustrates the schematic workflow of the current project.
References
- Nia HT, Munn LL, Jain RK. Mapping physical tumor microenvironment and drug delivery. Clinical Cancer Research. 2019; 25(7): 2024-6
- Steuperaert M, Debbaut C, Carlier C, De Wever O, Descamps B, Vanhove C, Ceelen W, Segers P. A 3D CFD model of the interstitial fluid pressure and drug distribution in heterogeneous tumor nodules during intraperitoneal chemotherapy. Drug delivery. 2019; 26(1): 404-15.
IBiTech researchers currently active on the project
Funding sources
- FWO Research project Kom op tegen Kanker – Grant G020919N (Wim Ceelen & Charlotte Debbaut)
- Stichting Tegen Kanker – Grant KW/1992/GIH/001/013 (Wim Ceelen)
Finalized PhDs within IBiTech
- Modeling of Drug Delivery and Transport during Intraperitoneal Chemotherapy (Margo Steuperaert)
- Algorithm Development and Protocol Optimization for Pharmacokinetic Modeling of Dynamic Contrast-Enhanced Magnetic Resonance Imaging (Dieter De Naeyer)
Relevant links