Hepatic Perfusion Modelling in Support of Liver Transplantation Strategies
Liver transplantation
Since liver transplantation became a true and successful treatment for liver failure, a substantial discrepancy arose between the supply of donor livers and the demand for liver transplantation during the last decades. As a consequence, there is a shortage of liver grafts for patients with liver failure and waiting lists are becoming longer. This urged research aiming to reduce the waiting list and increase the number of available liver grafts.
Machine perfusion
One of the proposed options to expand the donor pool is the use of organs that are currently uncommonly used for transplantation (e.g. livers from donation after circulatory death donors or extended criteria donors with contraindications such as liver steatosis, advanced age etc.). Compared to standard criteria organs (donation after brain death), these organs are more susceptible to ischemia-reperfusion injury, which is partly aggravated by the current gold standard of static cold storage. Consequently, machine perfusion (MP) preservation was proposed as a potential alternative preservation technique for donor organs, different from and better than static cold storage. MP can be done at different temperatures ranging from hypothermic (cold perfusion) to normothermic perfusion (mimicking normal in vivo temperatures and liver perfusion) [1]. Previous studies gave evidence of a better organ viability after MP preservation, especially in the case of extended criteria donor livers. Other advantages of MP include possibilities for longer preservation periods compared to static cold storage, monitoring the behavior and viability of the organ by measuring perfusion parameters etc. Nonetheless, liver MP also encountered some non-negligible disadvantages. As such, the two blood inlets of the liver may start to compete during MP [2]. Furthermore, it is known that the unique and complex hepatic microvasculature is prone to abnormal stressors which may arise due to hyperperfusion and lead to endothelial injury. In the end, the challenge is to define the most optimal perfusion settings to prevent microcirculatory damage while assuring sufficient and homogeneous perfusion. In this respect, it would be insightful to better understand the intrahepatic hemodynamics at both the macro- and microscale during MP and develop a liver perfusion model to test different settings.
Alternative transplantation techniques
Another possibility to circumvent the donor liver shortage is to use alternative transplantation techniques, such as living donor liver transplantation (LDLT) and split liver transplantation (SLT). In LDLT, a healthy living person donates a part of his liver to a patient with liver failure, while SLT stands for a deceased donor liver being split into two liver grafts to be transplanted into two recipients. The disadvantage of these transplantation techniques is the risk of developing the small-for-size syndrome (SFSS). SFSS is characterized by a liver that is too small compared to the patient’s body and may lead to serious complications or even liver graft failure. In this context, it is for instance not completely clear how much liver tissue can be safely removed from a living donor, or what the optimal resection planes are. More insight into the hemodynamics before and after partial hepatectomy is thus desired [3].
Research project focused on modelling liver perfusion in support of transplantation
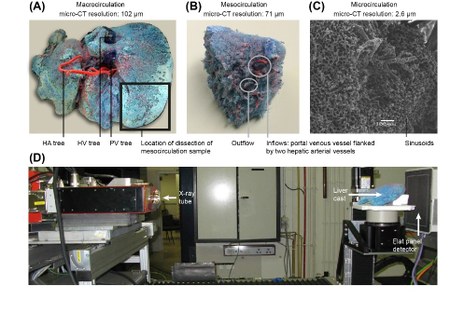
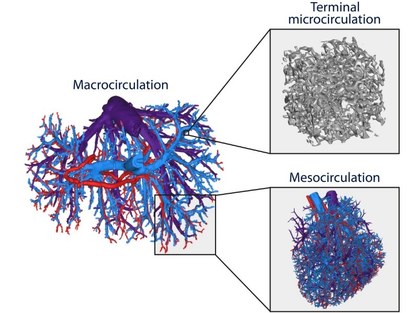
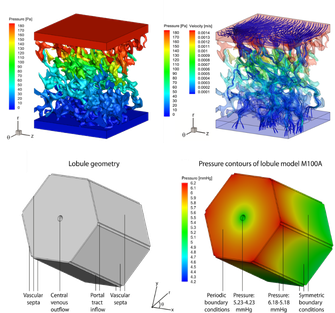
Since hepatic hemodynamics are shown to play an important role in different liver transplantation strategies as outlined above, this project focuses on clarifying and modelling hepatic hemodynamics and perfusion across different length scales. More specifically, a multi-level framework of models was developed to simulate hepatic perfusion at different scales. Hereby, both state-of-the-art experimental techniques (Fig. 1; e.g. vascular corrosion casting, micro-CT scanning, hypothermic machine perfusion…) and modelling techniques (image processing, electrical analog models and computational fluid dynamics (CFD)) were used to model liver perfusion at multiple length scales, ranging from the microcirculation up to the microcirculation (Fig. 2-3). This versatile framework was applied in the context of liver transplantation and resulted in unique 3D morphological and geometrical data on human and rat livers, as well as novel models and insights into the hemodynamic impact of machine perfusion preservation of donor livers, partial hepatectomy procedures (such as LDLT), and into the perfusion characteristics of the liver microcirculation. Doing so, we were able to find some missing pieces of the puzzle on liver perfusion. However, further research is necessary to reveal the complete picture.
Want to know more about this project?
Feel free to have a look at the publications related to this project, available at: https://biblio.ugent.be/list/0QMqAL7evnSNo90bEKxyFaOvn70WB
This project was done in close collaboration with the group of prof. dr. Diethard Monbaliu (Abdominal Transplantation Surgery, KULeuven/UZLeuven). Also other (inter)national research groups were involved as illustrated by the publication list above.
References
- Bellini, M. I., Nozdrin, M., Yiu, J., & Papalois, V. (2019). Machine Perfusion for Abdominal Organ Preservation: A Systematic Review of Kidney and Liver Human Grafts. Journal of clinical medicine, 8(8), 1221. https://doi.org/10.3390/jcm8081221
- Monbaliu DR, Debbaut C, Hillewaert WJ, Laleman WJ, Sainz-Barriga M, Pirenne J, Segers P. Flow competition between hepatic arterial and portal venous flow during hypothermic machine perfusion preservation of porcine livers. Int J Artif Organs. 2012 Feb;35(2):119-31. doi: 10.5301/ijao.5000038
- Gavriilidis P, Azoulay D, Sutcliffe RP, Roberts KJ. Split versus living-related adult liver transplantation: a systematic review and meta-analysis. Langenbecks Arch Surg. 2019 May;404(3):285-292. doi: 10.1007/s00423-019-01771-4
- Debbaut C, Segers P, Cornillie P, Casteleyn C, Dierick M, Laleman W, Monbaliu D. Analyzing the human liver vascular architecture by combining vascular corrosion casting and micro-CT scanning: a feasibility study. J Anat. 2014 Apr;224(4):509-17. doi: 10.1111/joa.12156
- Debbaut, Charlotte, Jan Vierendeels, Christophe Casteleyn, Pieter Cornillie, Denis Van Loo, Paul Simoens, Luc Van Hoorebeke, Diethard Monbaliu, and Patrick Segers. 2012. “Perfusion Characteristics of the Human Hepatic Microcirculation Based on Three-dimensional Reconstructions and Computational Fluid Dynamic Analysis.” Journal of Biomechanical Engineering-transactions of the Asme 134 (1).
- Debbaut, C., Vierendeels, J., Siggers, J. H., Repetto, R., Monbaliu, D., & Segers, P. (2014). A 3D porous media liver lobule model: the importance of vascular septa and anisotropic permeability for homogeneous perfusion. COMPUTER METHODS IN BIOMECHANICS AND BIOMEDICAL ENGINEERING, 17(12), 1295–1310.
IBiTech researchers currently active on the project
- Charlotte Debbaut (contact)
Funding sources
- Doctoral grant strategic basic research of the Agency for Innovation by Science and Technology in Flanders (IWT) – grant 101115 2011-2014 (Charlotte Debbaut)
- BOF starting grant – grant BOFSTA201909015 2019-2023 (Charlotte Debbaut)
Finalized PhDs within IBiTech
- Multi-level modelling of hepatic perfusion in support of liver transplantation strategies (Charlotte Debbaut)
