The Role of the Vasculature and Perfusion in Liver Pathologies
From a healthy liver towards liver disease
The liver is a complex and vital organ, responsible for more than 500 functions (e.g. the synthesis of proteins and breakdown of toxins) to maintain body homeostasis. Its ability to perform these functions is highly dependent on a well-functioning blood circulation and well-organized vascular architecture. Impairment of either one due to injury or disease may instigate a cascade of events, affecting the overall liver function and the functioning of the organism. Fortunately, the liver can withstand a certain degree of damage due to its unique capacity to regenerate tissue after injury. However, severe injury may eventually cause the liver to progress towards liver pathologies such as cirrhosis.
Liver cirrhosis: how is the liver vasculature and perfusion being affected during cirrhogenesis?
Cirrhosis is a detrimental condition of the liver, often attributed to an unhealthy lifestyle (e.g. alcohol abuse and fatty diet) or chronic viral infection (e.g. hepatitis B and C). It is characterized by a severe distortion of the hepatic architecture and mechanical properties, impairing the hepatic perfusion and function. Its genesis is insidious and may take up to many years before reaching a decompensated stage in which complications (e.g. ascites, variceal hemorrhage, jaundice, and encephalopathy) become clinically present. Once cirrhosis reaches the decompensated stage, it may culminate into liver insufficiency or liver cancer (e.g. hepatocellular carcinoma). Cirrhosis is ranked the twelfth leading cause of death worldwide. Unfortunately, cirrhosis often remains unnoticed until complications become clinically present [1].
Cirrhosis is still considered irreversible and early etiological treatment focuses on delaying disease progression and reducing complications. However, liver transplantation remains the only therapeutic option when liver insufficiency and/or complications occur. Consequently, it has become imperative to precisely assess the patient’s liver (function) to tailor the treatment. However, a patient-centered therapeutic approach remains challenging as the pathogenesis of cirrhosis is still not fully understood.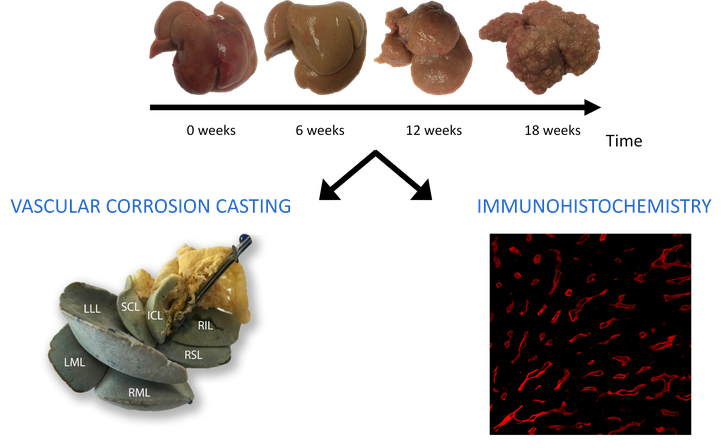
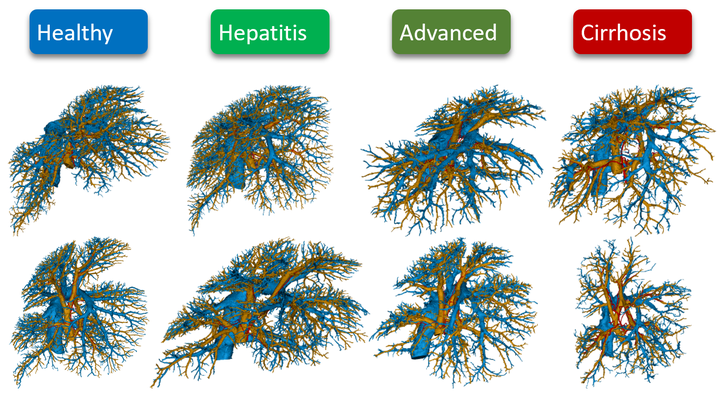
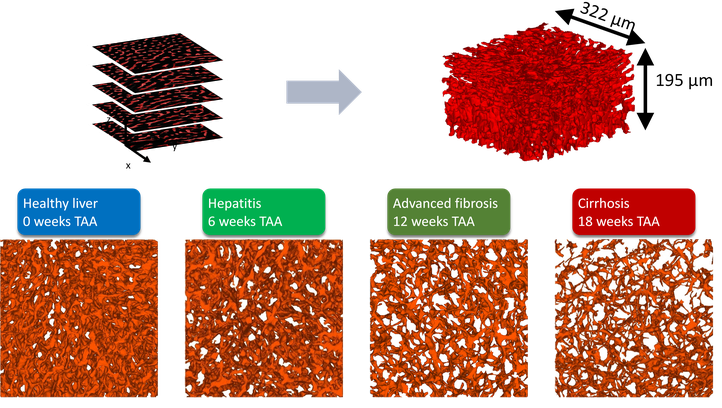
During this project, a methodological framework has been developed and implemented to quantify the vascular remodeling of the rat liver during cirrhogenesis using a combination of state-of-the-art experimental and data analysis techniques (vascular corrosion casting, micro-CT imaging, immunohistochemistry, clearing techniques, confocal laser scanning…) [2]. This allowed novel and unique 3D morphological data to be generated on the hepatic macro- and microcirculation throughout cirrhosis development [3]. These data then formed the basis for a computational model to simulate the rat blood circulation during cirrhogenesis. This computational model allowed for assessment of the hemodynamic consequences of cirrhogenesis and provided unique insights into the manifestation of portal hypertension [4]. We consider our work a step forward in unravelling the complex pathogenesis of cirrhosis. However, a lot of ground is yet to be uncovered.
Want to know more about this project?This project was done in close collaboration with the groups of prof. dr. Wim Laleman (Hepatology, KULeuven/UZLeuven) and prof. dr. Winnok De Vos (Laboratory of Cell Biology and Histology, UAntwerp). Also other (inter)national research groups were involved as illustrated by the publication list above.
Other topics
Next to liver cirrhosis, we work on research topics related to other liver diseases, e.g. locoregional drug delivery for the treatment of liver cancer. Another example is a project on Fontan patients in collaboration with the SickKids Hospital of Toronto and the University of Toronto (Canada). The Fontan procedure is performed to treat patients with a congenital heart disease resulting in a single functional heart ventricle. Hereby, the patient’s circulation is surgically modified in such a way that systemic venous blood flows directly to the pulmonary arteries, while bypassing the heart. Although this procedure improves the patient’s condition during childhood, the majority of adult patients develop complications including liver injury or disease (typically cirrhosis and hepatocellular carcinoma). Therefore, computational models of liver perfusion in these cases would be helpful to describe changes in the hepatic and intestinal circulation that are associated with the development of liver disease in Fontan patients. This will help to better identify patients at risk for significant liver and gut complications. A collaborative study on this topic is available at http://hdl.handle.net/1854/LU-8687579.
References
- Pellicoro, A., Ramachandran, P., Iredale, J. et al. (2014), Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol, 14: 181–194. https://doi.org/10.1038/nri3623
- Peeters, G., Debbaut, C., Laleman, W. et al. (2017), A multilevel framework to reconstruct anatomical 3D models of the hepatic vasculature in rat livers. J. Anat., 230: 471-483. https://doi.org/10.1111/joa.12567
- Peeters, G., Debbaut, C., Friebel, A., et al. (2018). Quantitative analysis of hepatic macro- and microvascular alterations during cirrhogenesis in the rat. Journal of anatomy, 232(3), 485–496. https://doi.org/10.1111/joa.12760
- Audebert C, Peeters G, Segers P, Laleman W, Monbaliu D, Korf H, Trebicka J, Vignon-Clementel IE, Debbaut C. Closed-Loop Lumped Parameter Modeling of Hemodynamics During Cirrhogenesis in Rats. IEEE Trans Biomed Eng. 2018 Oct;65(10):2311-2322. doi: 10.1109/TBME.2018.2793948
IBiTech researchers currently active on the project
- Charlotte Debbaut (contact)
Funding sources
- Doctoral grant strategic basic research of the Agency for Innovation by Science and Technology in Flanders (IWT) – grant 131446 2014-2017 (Geert Peeters)
- BASL research grant (Belgian Association for the Study of the Liver - 2015-2016 (Charlotte Debbaut & Geert Peeters)
- BOF starting grant – grant BOFSTA201909015 2019-2023 (Charlotte Debbaut)
Finalized PhDs within IBiTech
- Modelling the Degenerative Adaptation of the Liver Vasculature and Perfusion in Cirrhosis (Geert Peeters)
Relevant links