Atomic layer deposition
ALD basics
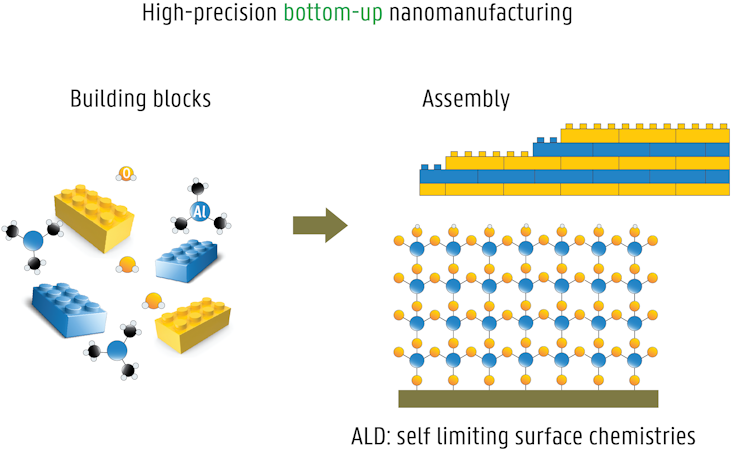
Atomic layer deposition (ALD) is a high-precision thin film deposition method, ideally suited for bottom-up nanomanufacturing. The material desired to be deposited as a thin film is packaged in a specifically designed molecule, typically a metalorganic precursor. The ALD process relies on alternating exposure of the growing film to the chemical precursor and a co-reactant (building blocks), which interact with the surface in a self-limiting manner (assembly). Each ALD cycle thus results in the deposition of sub-monolayers over the entire sample surface. ALD is an ideal method for coating nanostructures, because of its unique advantages:
- excellent conformality on 3D objects
- atomic level control of layer thickness and composition
The most important disadvantage is the limited deposition rate (at best a couple of nanometer / minute). However, this limitation becomes less relevant, since the coating of nanostructures only usually requires ultra-thin films.
The conventional ALD process, so-called thermal ALD, uses substrate heating to drive the surface chemical reactions. Typical ALD deposition temperatures lie in the range 100-350°C.
Plasma-enhanced ALD (PE-ALD) uses a plasma to generate reactive radicals, which are then used as a co-reactant in the process. Consequently, the plasma process depends less on the thermal energy available at the surface, which allows for film growth at lower temperatures and higher growth rates.
Molecular layer deposition (MLD) is a thin film deposition method closely related to ALD. However, where ALD is limited to exclusively inorganic coatings, the precursor chemistry in MLD is expanded to include organics in a controlled way and build up organic-inorganic hybrid materials.
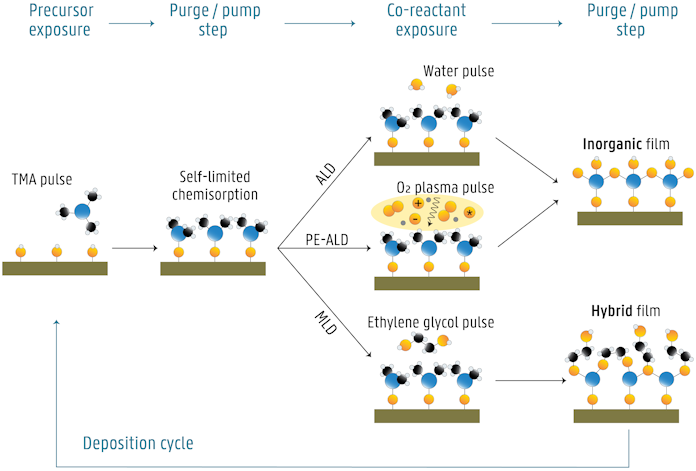
The figure below shows the example of ALD growth of Al2O3 via thermal ALD (a) and PE-ALD (b), and MLD growth of alucone (c). First, the Al-precursor, trimethylaluminum or TMA, is pulsed to the reactor and it reacts with the hydroxyl surface species. Once all adsorption sites have been consumed, no more precursor will react. The excess precursor and possible byproducts are removed from the reactor, either by purging with inert gas or by pumping. Next, the co-reactant is introduced, which reacts with the precursor molecules anchored to the surface. This surface reaction is again self-saturating, and is followed again by purging/pumping of the reactor. In the ideal case, a monolayer of Al2O3 (ALD) or alucone (MLD) is formed, and the layer is terminated with surface groups that can react with the Al-precursor again. Therefore, the deposition cycle can be repeated, thereby building up inorganic or hybrid films one layer at a time.
Literature:
Puurunen, Riikka L. “Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process” JOURNAL OF APPLIED PHYSICS 97, 121301 (2005). DOI: 10.1063/1.1940727
Malygin, Anatolii A., et al. “From V. B. Aleskovskii's “Framework” Hypothesis to the Method of Molecular Layering/Atomic Layer Deposition” CHEMICAL VAPOR DEPOSITION 21 (2015): 216-240. DOI: 10.1002/cvde.201502013
Ahvenniemi, Esko, et al. “Review Article: Recommended reading list of early publications on atomic layer deposition—Outcome of the “Virtual Project on the History of ALD"” Journal of Vacuum Science & Technology A 35, 010801 (2017). DOI: 10.1116/1.4971389
ALD conformality
As mentioned above, one of the key selling points of ALD is its conformality: the ability to coat complex 3D substrates with a film of uniform thickness. The conformality is intrinsic to the nature of ALD. Conformal ALD coatings can be applied to wafers with nanoscale 3D features, porous substrates, membranes, powders, etc.
A direct and macroscopic illustration of the excellent conformality of ALD is shown below. Tissue paper was coated with aluminum oxide using ALD, rendering a hydrophobic surface. After combustion of the cellulose template with a lighter, a network of hollow alumina tubes is left behind. This experiment clearly demonstrates the potential of ALD for coating porous and fibrous materials.
Making a tissue hydrophobic ...
"Original" tissue paper
Al2O3 ALD coated tissue paper
Making a tissue fire resistant ...
"Original" tissue paper
Al2O3 ALD coated tissue paper
We have developed two experimental approaches to investigate the conformality of ALD processes:
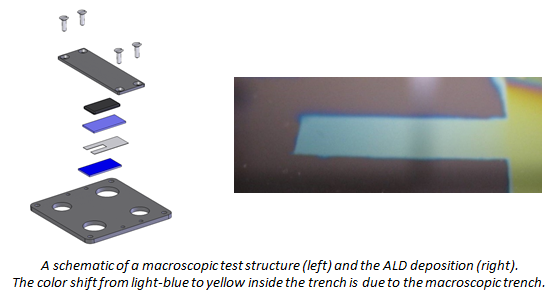
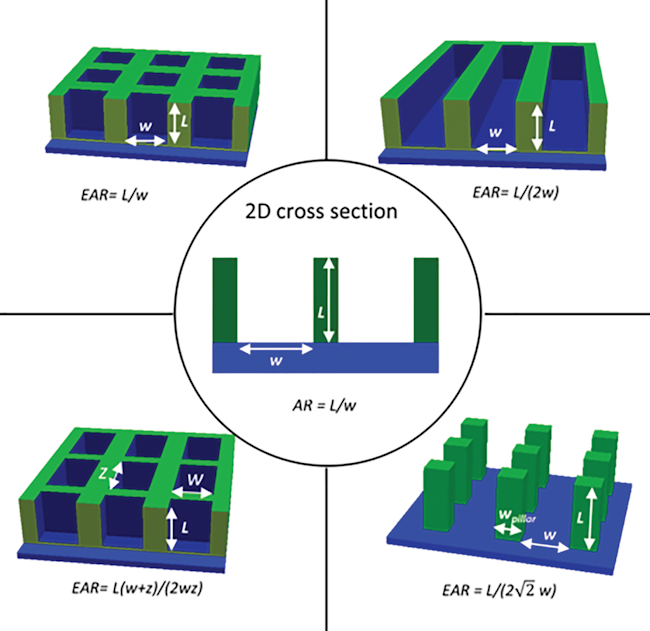
- When performing ALD at low pressures (i.e. in a pump-type reactor), molecular flow persists up to millimeter sized structures, and therefore macroscopic high-aspect-ratio test structures can be used for quantifying the conformality of ALD processes. This simple and efficient approach allowed us to investigate the conformality of plasma enhanced ALD [1,2] and to characterize and model the effect of the sticking probability of precursor molecules on the conformality [3].
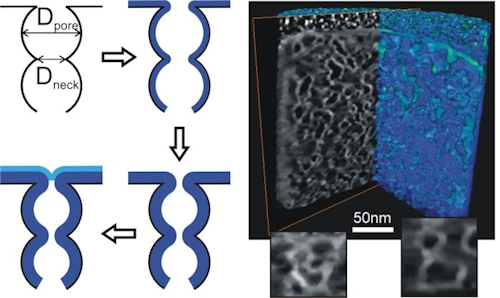
- Nanoporous thin films were used as model systems to explore the conformality of ALD inside nanopores [4,5].
At CoCooN, we have more than 10 years of experience in simulation of conformality of ALD. To this aim, we have developed our own Monte Carlo Code [6] and our expertise culminated in a recent, well-received review paper on conformality in ALD [7].
Publications:
- Dendooven, Jolien, et al. “Conformality of Al2O3 and AlN Deposited by Plasma-enhanced Atomic Layer Deposition.” JOURNAL OF THE ELECTROCHEMICAL SOCIETY 157, 4 (2010): G111–G116. DOI: 10.1149/1.3301664
- Musschoot, Jan, et al. “Conformality of Thermal and Plasma Enhanced Atomic Layer Deposition on a Non-woven Fibrous Substrate.” SURFACE & COATINGS TECHNOLOGY 206, 22 (2012): 4511–4517. DOI: 10.1016/j.surfcoat.2012.02.038
- Dendooven, Jolien, et al. “Modeling the Conformality of Atomic Layer Deposition: The Effect of Sticking Probability.” JOURNAL OF THE ELECTROCHEMICAL SOCIETY 156, 4 (2009): P63–P67. DOI: 10.1149/1.3072694
- Dendooven, Jolien, et al. “In Situ X-ray Fluorescence Measurements During Atomic Layer Deposition: Nucleation and Growth of TiO2 on Planar Substrates and in Nanoporous Films.” JOURNAL OF PHYSICAL CHEMISTRY C 115, 14 (2011): 6605–6610. DOI: 10.1021/jp111314b
- Dendooven, Jolien, et al. “Tuning the Pore Size of Ink-Bottle Mesopores by Atomic Layer Deposition.” CHEMISTRY OF MATERIALS, vol. 24, no. 11, 2012, pp. 1992–94. DOI: 10.1021/cm203754a
- Cremers, Véronique, et al. “Monte Carlo Simulations of Atomic Layer Deposition on 3D Large Surface Area Structures : Required Precursor Exposure for Pillar- Versus Hole-type Structures.” JOURNAL OF VACUUM SCIENCE & TECHNOLOGY A 35, 1 (2017). DOI: 10.1116/1.4968201
- Cremers, Véronique, et al. “Conformality in Atomic Layer Deposition : Current Status Overview of Analysis and Modelling.” APPLIED PHYSICS REVIEWS, vol. 6, no. 2, 2019. DOI: 10.1063/1.5060967
ALD research @ COCOON
ALD research at CoCooN is focused on the following topics:
- As shown in the previous section, one of our research topics is headed into experimental studies and modelling of conformality of ALD.
- CoCooN has developed several tens of ALD processes. See below in the interactive application.
- CoCooN is well-known in the worldwide ALD community for its in situ characterization capabilities during film growth, including ellipsometry, mass spec, infrared spectroscopy, etc.
- The CoCooN research group has pioneered the use of synchrotron-based X-ray techniques for in situ characterization during ALD.
- The group patented a rotary reactor system enabling plasma-enhanced ALD on particles. Read more about our coating technologies for particles.
- ALD is being investigated as a coating technique in several applications.