Design and systematic study of imidazole based DNAzymes
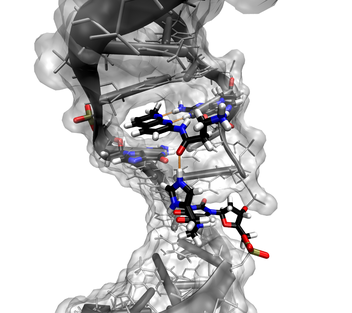
We here explore the use of a 14mer DNA duplex as a scaffold for the precise and predictable positioning of catalytic functionalities.
Given the ubiquitous participation of the histidine-based imidazole group in protein recognition and catalysis events, single histidine-like modified duplexes were investigated.
Tethering histamine to the C5 of the thymine base via an amide bond, allows the flexible positioning of the imidazole function in the major groove.
The mutual interactions between the imidazole and the duplex and its influence on the imidazolium pKa are investigated by NMR and moleclar dynamics.
Lars.Verdonck@UGent.be