Dr. Laia Miret Casals
CV
Laia Miret Casals received a Ph.D. in Organic Chemistry at the University of Barcelona under the mentorship of Prof. Fernando Albericio and Prof. Antonio Zorzano, where she first fell in love with chemical biology while exploring small molecules for modulating biological targets. After her PhD, she did postdoctoral studies on the development of bicyclic peptides drugs for the treatment of respiratory infectious diseases with Almirall Pharma/University of Barcelona. Then, she moved to The University of Queensland with A/Prof. Markus Muttenthaler to develop molecular probes to study memory formation. She was a postdoctoral assistant in Prof. Annemieke Madder’s group at Ghent University.
Her research focused on applying the furan cross-link technology for protein-protein and ligand-GPCR interactions to develop novel diagnostics and therapeutics tools.
Research Project
Furan-oxidation mediated cross-link technology: from in vitro analysis of protein-protein interactions to covalent GPCR-ligand interactions on live cells
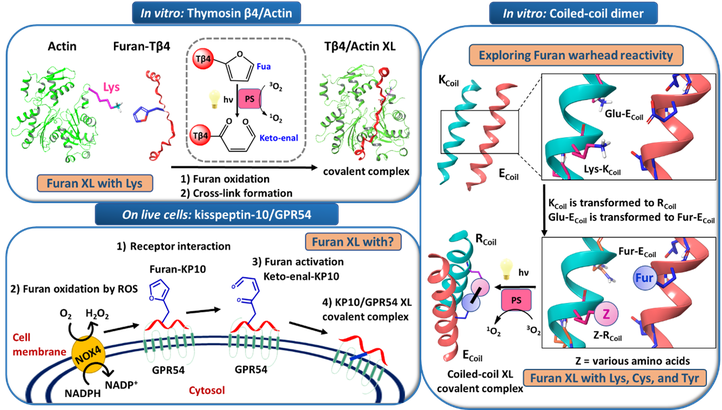
Our research group has developed a cross-linking technology based on furan chemistry. Here, we describe the application of this fast and highly efficient furan-oxidation mediated technology to protein-protein1, coiled-coil peptide dimer2, and peptide-protein3 interactions.
Initially, we studied the weak and dynamic protein-protein interaction between actin and Tß4 that regulates the polymerization of actin and keeps actin in the monomeric form. Furan-armed-Tß4 analogues were shown to efficiently cross-link to monomeric actin upon singlet oxygen generation1. The cross-link in the Tß4-actin covalent complex was to involve Tß4-Fua24 and Actin-Lys611. A coiled-coil peptide dimer was used as a model system to explore furan reactivity, we described for the first-time reaction of the activated furan warhead with cysteine and tyrosine, besides the previously reported lysine, thus enhancing the versatility of the furan cross-link technology by the possibility to target different amino acids2. The furan-oxidation mediated cross-link technology was further optimized to enable cross-linking of furan-modified peptide ligands to GPCR proteins on live cells relying on the spontaneous endogenous oxidation of the furan moiety. We studied the neuropeptide kisspeptin-10 and its G-protein coupled receptor GPR54. We described selective cross-linking of a furan-modified kisspeptin-10 analogue to its membrane receptor GPR54 in live cells, with no toxicity and high efficiency3,4.
[1] Miret-Casals, L; Vannecke, W; Hoogewijs, K; et al. Chem. Comm., 2021, 57, 6054-6057. [2] Miret-Casals, L; Van de Putte, S; Aerssens, D; et al. Frontiers in Chemistry, 2022, DOI: 10.3389/fchem.2021.799706. [3] Vannecke, W; Van Troys, M; Ampe, C; Madder, A. ACS Chem. Biol. 2017, 12, 2191-2200. [4] Van Troys, M; Vannecke, W; Ampe, C; Madder, A. G Protein-Coupled Receptor Signaling/Methods and Protocols. 2019, 1947, 81–102.