Research
Furan Crosslink Technology
A biocompatible, covalent cross-linking methodology which displays a suitable balance between reactivity, proximal action, and specificity.
Bioconjugation
The combination of two entities (small-molecules, proteins, peptides or nucleic acids) leads to bioconjugates bearing new functionalities which can be exploited for several purposes (e.g. therapeutics).
Nucleic Acid Cancer Biomarker Detection
Modified oligonucleotides are developed for low-level detection of Nucleic Acid Cancer Biomarkers using the novel photoelectrochemistry-based ‘SOCan technology' .
Peptide and protein conjugates
Peptides and proteins need conjugation to property-enhancing moieties to improve their therapeutic performance.
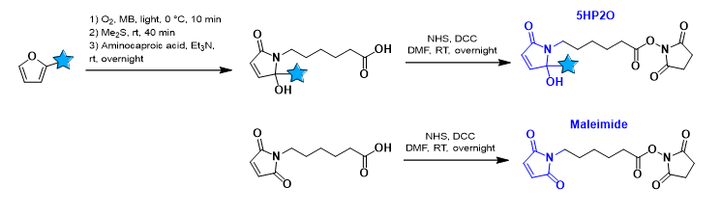
Discover our 5HP2O building blocks available through ![]() .
.
PNA (Peptide Nucleic Acids)
Peptide Nucleic Acids are easily synthesised DNA/RNA Analogues with remarkable physicochemical properties.
PAST PROJECTS
Aptamer Biosensing
Aptamers, a promising alternative for antibodies in therapeutics and diagnostics. Let’s try understanding the secrets of an aptamer-target interaction.
Artificial Receptors
The SynAb receptors are a solution to substitute antibodies by taking advantage of ion-ionophore interactions. The synthetic modular approach of SynAb receptors benefits the adaptation of this concept to create valuable technologies.
Bioconjugation
Antibody-drug conjugates (ADCs) are novel therapeutic constructs where the small-molecule drug is delivered specifically to relevant cells by exploiting antibodies. Key to this modality is the chemical bond between the drug and the antibody and its synthetic formation. Generally, the combination of two entities (e.g. small-molecule, peptide, protein) in one construct (=conjugate) can be performed either by chemical or enzymatic bioconjugation or by self-assembly. Chemical bioconjugation methodology either exploits natural amino acids (e.g. cysteine or lysine) or biorthogonal handles (e.g. azide) which however often requires a tedious incorporation of the handle into the biomacromolecule. All three approaches have a different applicability and lead to a vast variety of chemical connections bearing different properties (e.g. in terms of stability).
Notably, the sulfohydryl (-SH) residue of cysteine represents an excellent moiety regarding its reactivity under physiological conditions, its low abundance in proteins and the possibility for manual insertion. As a result, most approved ADCs are based on thiol-maleimide chemistry (see scheme). However, these conjugates lack in predictability of stability as they are unstable under certain circumstances , which results in the loss of the payload (small molecule drug in the case of ADCs).
The OBCR group recently developed a novel bioconjugation methodology based on 5-Hydroxy-Pyrrolone building blocks (5HP2O) which can be considered as more stable alternatives for classical maleimides. Advantages of 5HP2O conjugation chemistry include predictability, irreversibility and multi-functionalisation. Currently we are interested in applying this methodology for the bioconjugation between antibody (analogues) and DNA/PNA.
Relevant Papers:
- Triazolinedione protein modification: from an overlooked off-target effect to a tryptophan-based bioconjugation strategy.
- 5-Hydroxy-Pyrrolone Based Building Blocks as Maleimide Alternatives for Protein Bioconjugation and Single-Site Multi-Functionalization.
- When a methyl makes the difference: hydrolysis of 5-methylfuran-2-yl to 2,5-dioxopentanyl allows for stable bioorthogonal proximity-induced ligation.
Current Team Members working in that area:
Past Team Members who worked in that area:
CLiPs
in close collaboration with the NMRSTR group of Prof. Dr. J. Martins
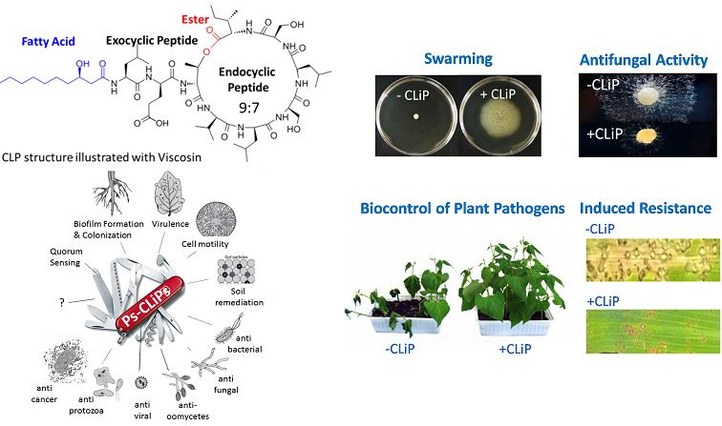
Cyclic lipodepsipeptides (CLiPs) are produced by bacteria such as Pseudomonads. These Pseudomonas spp. belong to a genus of Gram-negative γ-proteobacteria that are able to colonise a wide variety of environments. To date, over 120 structurally distinct Ps-CLiPs have been described, however they all adhere to a common chemical blueprint as depicted in the figure. Essentially, they are composed of fatty acid (lipo) acetylated at N-terminus of small peptide chain and cyclised (cyclic) via side-chain-to-tail macrolactonisation (depsi). They were produced via non-ribosomal peptide synthases (NRPSs). This NRPS assembly line accommodates the incorporation of non-proteinogenic amino acids and, accordingly, CLiPs typically consist of a mixture of L- and D- amino acids, thereby reducing their susceptibility to enzyme degradation by for instance peptidases.
This class of compounds has gained a huge interest for their broad range of biological activities for instance anti-microbial, anti-fungal, anti-viral and anti-cancer properties. In addition, a multitude of studies have established the beneficial activities of CLiPs for agricultural applications. they can promote plant growth and they can also trigger the plant defence response against pathogens providing protection against diseases.
Nevertheless, their mode of action has not yet been clearly discovered. To understand the mechanism of action and to engage into structure-function studies, their precise structure including stereochemistry needs to be established. To achieve this, we have developed a suitable synthesis route as well as an approach that relies on matching the NMR spectral profile of the natural compound to a library of synthetic homologues.
Relevant Papers:
- Breaking cycles : saponification‐enhanced NMR fingerprint matching for the identification and stereochemical evaluation of cyclic lipodepsipeptides from natural sources
- An nuclear magnetic resonance fingerprint matching approach for the identification and structural re-evaluation of Pseudomonas lipopeptides.
- First total synthesis of WLIP: on the importance of correct protecting group choice.
Current Team Members working in that area:
Past Team Members who worked in that area:
Furan Crosslink Technology
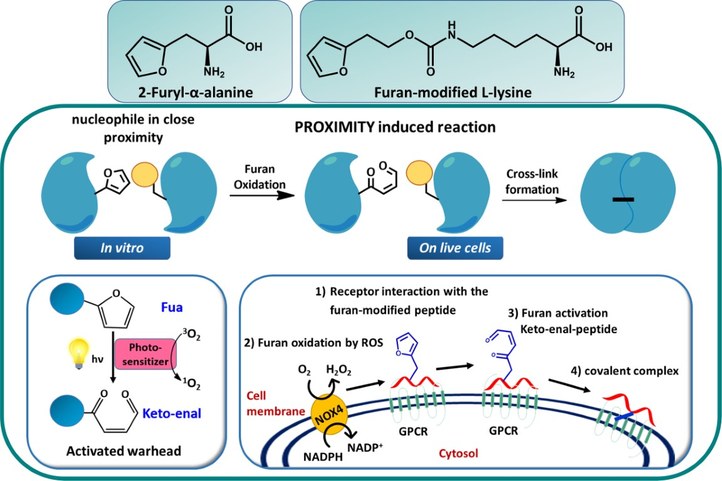
Peptide–protein and protein-protein interactions play key roles in biological processes. Therefore, approaches to target proteins in a covalent way provide useful analytical tools for chemical biology and could lead to potential applications in therapeutic development. Within OBCR we developed a novel cross-link technology applied to coiled-coil peptide dimers, peptide–protein, and protein–protein interactions, based on the introduction of an unnatural, furan-containing amino acid in a peptide or protein-ligand, followed by its oxidation into an electrophilic moiety upon the action of reactive oxygen species (ROS) or visible light irradiation in the presence of a photosensitizer (PS). Synthetic peptides and small proteins can be equipped with a furan moiety, through the introduction of an unnatural amino acid, such as 2-furyl-L-alanine (Fua) or furan-modified L-lysine, by solid-phase peptide synthesis or genetic code expansion, respectively. Since Fua is isosteric with histidine and isoelectronic with tyrosine, it exerts only minimal structural perturbations and results, upon appropriate positioning, in unaltered biological activity. The inserted furan moiety forms a ‘caged warhead’ as covalent bond formation is triggered by oxidation of furan into the corresponding keto–enal. This flexible reactive moiety scans its immediate surroundings for nucleophiles in the bound partner and will only form a specific covalent bond when this nucleophile is sufficiently proximal. Importantly, both amine and thiol species are suitable nucleophiles for activated furan.
Relevant Papers:
- Furan-based (photo)oxidation reactions and their application in nucleic acid and protein targeting
- Furan oxidation based cross-linking: a new approach for the study and targeting of nucleic acid and protein interactions.
- Sequence specific furan based DNA crosslinking with visual light.
- Furan-modified oligonucleotides for fast, high-yielding and site-selective DNA inter-strand cross-linking with non-modified complements.
- Crosslinking furan-modified kisspeptin-10 with the KISS-receptor: NOX does the trick.
Current Team Members working in that area:
Past Team Members who worked in that area:
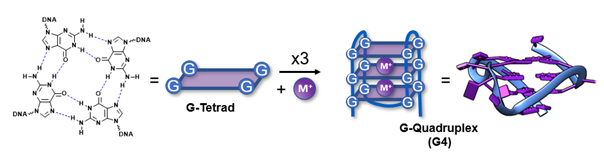
G-Quadruplexes
Guanine-rich nucleic acid sequences have the inherent propensity to interact with each other and fold into G-Quadruplexes (G4s). These non-canonical secondary structures consist of stacked G-tetrads and are formed upon interaction of four guanines, stabilized by Hoogsteen hydrogen bonds and cations. This naturally occurring phenomenon has demonstrated to be widespread throughout the whole genome and transcriptome, such as at the end of the telomeres, in promotor regions of proto-oncogenes, in mRNA and even ncRNAs. By inducing or interrupting the G4-folding, one could regulate biological relevant processes like gene expression or translation, and therefore, these structural peculiarities are considered important targets in anti-cancer research.
Relevant Papers:
- Development of a His-Tag-mediated pull-down and quantification assay for G-quadruplex containing DNA sequences
- Locking up G-quadruplexes with light-triggered staples leads to increased topological, thermodynamic, and metabolic stability
- Exploiting G-quadruplex-DNA damage as a tool to quantify singlet oxygen production
- A red light-triggered chemical tool for sequence-specific alkylation of G-quadruplex and I-motif DNA.
- Beyond Small Molecules: Targeting G-Quadruplex Structures with Oligonucleotides and Their Analogues.
- Teaching Photosensitizers a New Trick: Red Light-Triggered G-Quadruplex Alkylation by Ligand Co-localization.
Current Team Members working in that area:
Past Team Members who worked in that area:
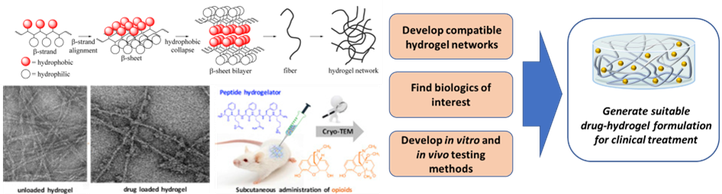
Hydrogels
Hydrogels obtained from oligopeptides that are composed of natural amino acids are of particular interest. Not only do these gels require a small amount of product, they are also not toxic and fully broken down in the body. Furthermore, these peptide-based hydrogels can be injected subcutaneously, allowing the drug to be released slowly, resulting in a more constant plasma drug concentration in function of time. Therefore, these injectable, drug loaded hydrogels could provide a solution for patients that need to take medications for prolonged periods of time, especially if they need to make frequent visits to the hospital for treatment by injection.
Relevant Papers:
- Peptide hydrogels as slow-release formulations of protein therapeutics : case study of asparaginase-loaded hydrogels
- Biodegradable amphipathic peptide hydrogels as extended-release system for opioid peptides.
- In vivo imaging of the stability and sustained cargo release of an injectable amphipathic peptide-based hydrogel.
Current Team Members working in that area:
Past Team Members who worked in that area:
Nucleic Acid Cancer Biomarkers
The interuniversity research project SOCan aims to develop a ground-breaking photoelectrochemistry-based ‘SOCan technology’ for the fast detection of clinically relevant levels of nucleic acid cancer biomarkers in patient samples. A consortium of three organizations receives a subsidy of 3 million euro for this iBOF project.
Cancer refers to the uncontrollable growth and spread of body cells, affecting tissue and organs, and is mainly linked to cellular DNA changes. Cancer can affect anyone at any time, with challenges in both diagnosis and treatment. In recent decades, molecular biomarker testing became more important to enable oncologists to specifically diagnose cancers and to accurately match therapies to individual patients. However, routine biomarker detection technologies come with significant challenges: high cost, complexity, requiring specialist knowledge, low miniaturization potential and limited accessibility.
SOCan will address these bottlenecks in current molecular testing methods by developing, optimizing and validating an innovative solution to detect DNA alterations and RNA, selectively and specifically. The technology relies on singlet oxygen-based photoelectrochemistry and combines (i) photosensitizers that produce singlet oxygen upon illumination; (ii) capture and detection probes; (iii) redox reporters; and (iv) magnetic beads for immobilization and enhancing sensitivity. Such a platform is highly sensitive, robust, fast, easy to setup and allows for re-use.
Current Team Members working in that area:
- Ahmet Colaker
- Batuhan Baytekin
- Sudip Ghosh
Oligonucleotide therapies
Oligonucleotides and their analogues are used in several therapies for treating genetic disorders. Despite their promise, they are associated with poor stability, suboptimal delivery and off-target effects. With the support of the Marie Skłodowska-Curie Actions programme, the ON-TRACT project aims to unlock the full potential of oligonucleotide therapies for treating lung cancer, B-cell malignancies, and chronic inflammation. The project brings together experts across academia, industry, and healthcare who will focus on sustainable synthesis and ethical testing using organoids. They will also explore advanced delivery systems such as monoclonal antibodies and inhalers. The ultimate goal is to lay the foundation for responsible, innovative therapies for many severe diseases.
PNA
Peptide nucleic acids (PNAs) are synthetic DNA mimics, composed of a neutral polyamide backbone to which nucleobases are attached, that are normally synthesized via solid phase synthesis. As a consequence of its uncharged backbone, there is no electrostatic repulsion upon hybridization of PNA to DNA/RNA, leading to more thermodynamic stable duplexes with DNA and RNA than DNA/RNA themselves. Additionally, this synthetic analogue has demonstrated to be resistant against proteases and nucleases. Besides the enzymatic resistance, PNAs are also thermally and chemically stable in conditions that normal oligonucleotides would degrade. Altogether, PNA could represent itself as a useful and easy synthesizable alternative for regular oligonucleotides.
Relevant Papers:
- Furan-modified PNA probes for covalent targeting and ligation of nucleic acids
- Synthesis and structure–activity relationship of peptide nucleic acid probes with improved interstrand-crosslinking abilities: application to biotin-mediated RNA-pulldown.
- Visible-light triggered templated ligation on surface using furan-modified PNAs.
Current Team Members working in that area:
Past Team Members who worked in that area:
PAST PROJECTS
Aptamer Biosensing
in close collaboration with the NMRSTR group of Prof. Dr. J. Martins
Aptamers are short, single stranded oligonucleotides (DNA or RNA) raised against a specific target by a process called SELEX (Systematic Evolution of Ligands by Exponential enrichment). These so-called affinity ligands are often compared to natural protein-based antibodies because they bind and interact with their target through a structural recognition process. However, their synthetic production, conformational change upon binding, renaturation capabilities, nonimmunogenic properties and ability to be modified makes them very promising alternatives for antibodies and their applications.
A typical example of those applications are biosensors, where the aptamer will take on the role as target recognition element (bioreceptor). With their design based on a conformational change of the aptamer upon binding with its target, these sensors are capable of detecting the binding process between aptamer and target.
Despite these aptamers being very promising, only a few made it as real world applications. To tackle this problem, optimisation of aptamer-target interactions and studying the underlying molecular mechanisms taking place during such an aptamer-target interaction are topics of our projects.
Relevant Papers:
Past Team Members who worked in that area:
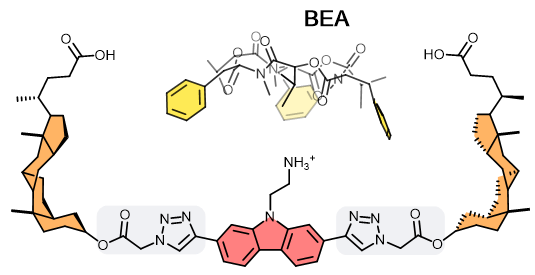
Artificial Receptors
Artificial synthetic antibody (SynAb) receptors are well-defined organic molecules that display high affinities to a target analyte. These use strong intramolecular interactions such as hydrogen bonds, reversible covalent bonds, and electrostatic forces. In the OBCR group, we design SynAb receptors for ionophoric cyclic depsipeptides (CDPs). These share a common blueprint combining a hydrophobic surface with a pronounced binding affinity towards cations like K+ and NH4+. These properties form the foundation for SynAb receptors developed to target CDPs. The SynAb receptor’s basic structure comprises 1) a carbazole ammonium cation component that binds to the ionophore CDP; 2) two steroid components that create a lipophilic cavity, increasing the CDP’s affinity to the receptor; 3) two spacers between components 1 and 2 that regulate the cavity size and its degree of rigidity/flexibility. Given its modular synthetic nature, the SynAb receptor’s binding affinity/selectivity toward cyclic toxins can be enhanced and modulated by creating differently-sized cavities. Furthermore, SynAbs are compatible with organic solvents and offer the freedom to chemically derivatise to add reactive handles for their implementation as affinity reagents in analytical assay development.