Laure Tack
CV
Laure Tack obtained her Master of Science in chemistry summa cum laude at Ghent University in 2020. Her master dissertation, titled ‘Synthesis and analysis of solvatochromic 7-aryl-3-methoxychromone based 2’-deoxyribonucleotides for sensing changes in the local microenvironment’, was performed abroad as an Erasmus project under the supervision of Prof. Dr. Michal Hocek at the Institute of Organic Chemistry and Biochemistry in Prague.
She completed her PhD as a FWO fundamental research PhD fellow in the OBCR group under the supervision of Prof. Dr. Annemieke Madder and in close collaboration with Prof. Dr. Jan Gettemans and Prof. Dr. Marleen Van Troys.
Her research consisted of exploiting crosslinking methodologies based for covalent trapping of cell-surface receptors and detection/labelling of protein modifications.
Research Project
Covalent ligands on the rise! In the current drug design, mainly based on using classical non-covalent binders, major challenges concerning the dramatic increase of drug resistance, mutations and toxicity, arise. Covalent inhibitors are a promising tool to address some of these problems due to their enhanced biochemical efficacy, less frequent dosing and improved pharmacokinetic parameters. Therefore, fundamental research towards the application of covalent crosslinking methodologies in a bio(-organic) context is required.
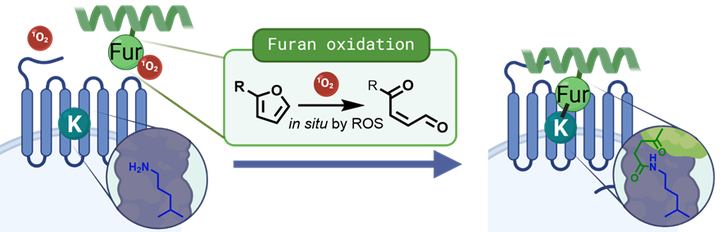
The furan oxidation technology, illustrated in figure 1, is a selective, spatiotemporally controlled covalent crosslinking methodology already applied on DNA, RNA and small protein-protein interactions. In this PhD research, this crosslinking methodology is applied to trap peptide-protein interactions at the cell surface in order to pave the pathway towards covalent peptide/protein therapeutics.
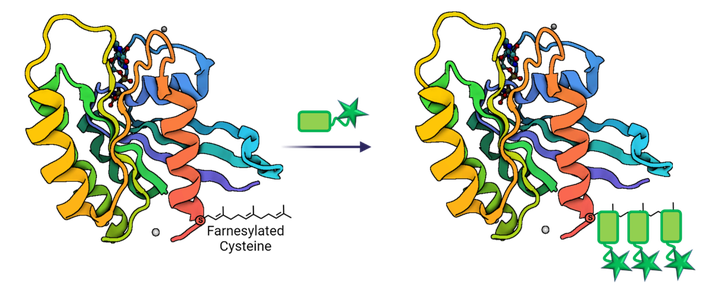
Next to targeting cell-surface receptors, the PhD research also focusses on applying covalent labelling methodologies in order to detect post-translational protein modifications, such as protein farnesylation. The latter is being upregulated in various diseases, for example Alzheimer disease, and is up until now only labelled by applying metabolic incorporation of isoprenoid analogues decorated with a fluorescent or biotin moiety or bio-orthogonal group, followed by click chemistry. Nevertheless, the time-consuming, metabolic introduction of a modified farnesyl moiety gives labelling of the native farnesyl group a substantial advantage (figure 2).