Ion-exchange nanofiber membranes for electrochemical water treatment
Solving environmental challenges with nanofibers
The availability of clean drinking water and the safe disposal of wastewater are major concerns in today’s society. Electrochemical techniques offer a way for localized water treatment which can save many lives in third world countries. To improve the efficiency of these systems, the use of nanofiber ion-exchange membranes is investigated in this research. Results showed excellent chemical resistance and fouling performance, outperforming that of commercial membranes.
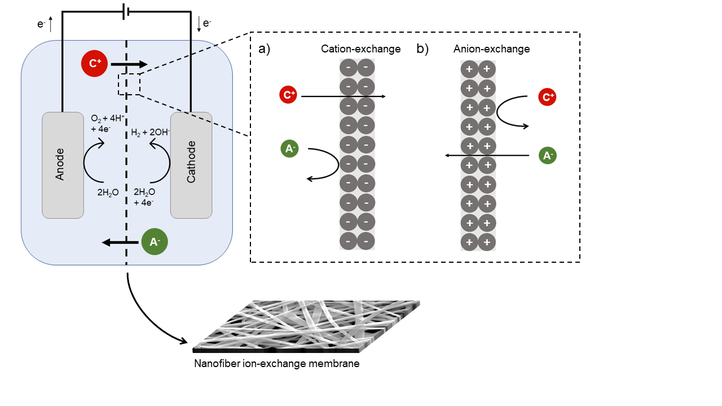
In view of today’s challenges in clean water depletion and wastewater management, electrochemical water treatment processes are increasingly applied for e.g. desalination of brackish water, recovery of resources and energy production from waste streams, and disinfection of wastewater. These electrochemical processes typically make use of an ion-exchange membrane (IEM) in between the anodic and cathodic compartment. The choice of material for this membrane is crucial for the performance of the cell and its performance is often the key bottleneck. Over the last decade, research has been focused on the production of ion-exchange nanofibers as membrane material due to their outstanding ionic properties as a result of their specific morphology. Nanofiber membranes are known to have a large specific surface area, flexibility, high porosity and interconnected pores. Different strategies are applied for the production and structural design of these ion-exchange nanofiber membranes. Nanofibers with an ion-exchange functionality can be produced by either pre- or post-functionalization methods, combined with electrospinning. Depending on the application, these nanofiber mats can be used as such, or further membrane processing is possible to improve the dimensional stability, typically by adding a pore-filling matrix in between the nanofibers.
By producing IEMs from hybrid nanofibrous membranes containing both organic and inorganic parts, a wide range of different membrane properties can be obtained by altering the molecular structure, which is the focus of this research. This results in IEMs with high thermal and chemical resistance as well as tunability towards a.o. mechanical properties and hydrophobicity for the use in harsh environmental conditions. For example, sulfonated silica-based nanofiber cation-exchange membranes (CEMs) have shown to offer a promising solution to the current issues of IEMs, due to their superior chemical resistance and self-cleaning properties after fouling.
Further information
Acknowledgements
- FWO grant 1S84322N
- BOF grant BOF19/24J/102
Contact
Prof. dr. ir. Karen De Clerck (Karen.DeClerck@UGent.be)