Sophie Janssens - DC-RIDDLE
Description of the PI

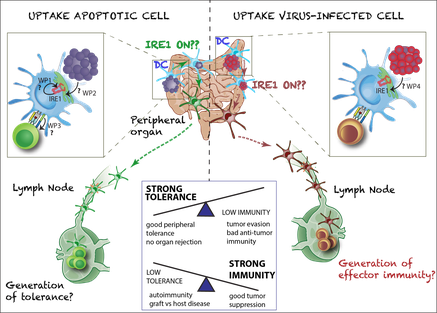
Her research team focuses on the role of the unfolded protein response in immune cells. They discovered that amongst all immune cells in our body, one subset of conventional dendritic cells (DC), the cDC1s, shows a particular high activation of the IRE1/XBP1 branch in steady state. This does not lead to the activation of a canonical XBP1 gene signature, but appears linked to their specific function in dead cell-derived antigen presentation. The Janssens lab is currently unraveling how the IRE1alpha branch in DCs shapes their immune responses, both in steady state as well as during viral infections.
Description of the project
Specifically, we found that one of the branches of the unfolded protein response (UPR), the IRE1/XBP1 signaling axis, is constitutively active in murine dendritic cells (cDC1s), without any signs of an overt UPR gene signature. Based on preliminary data we hypothesize that IRE1 is activated by apoptotic cell uptake, orchestrating a metabolic response from the ER. This entirely novel physiological function for IRE1 entails a paradigm shift in the UPR field, as it reveals that IRE1’s functions might stretch far from its well-established function induced by chronic ER stress. The aim of my research program is to understand by which signals IRE1 is activated in DCs and how it might affect the switch between tolerogenic and immunogenic maturation. To this end, we will dissect its function in vivo both in steady-state conditions and in conditions of danger (viral infection models). I envisage that my research program will not only have a large impact on the field of DC biology and apoptotic cell clearance, but might also yield new insights in diseases like autoimmunity, graft versus host disease or tumor immunology, all associated with disturbed balances between tolerogenic and immunogenic responses.
Contact
e-mail
publications